-
Vector Platform
-
CMC Platform
-
Manufacturing Platform
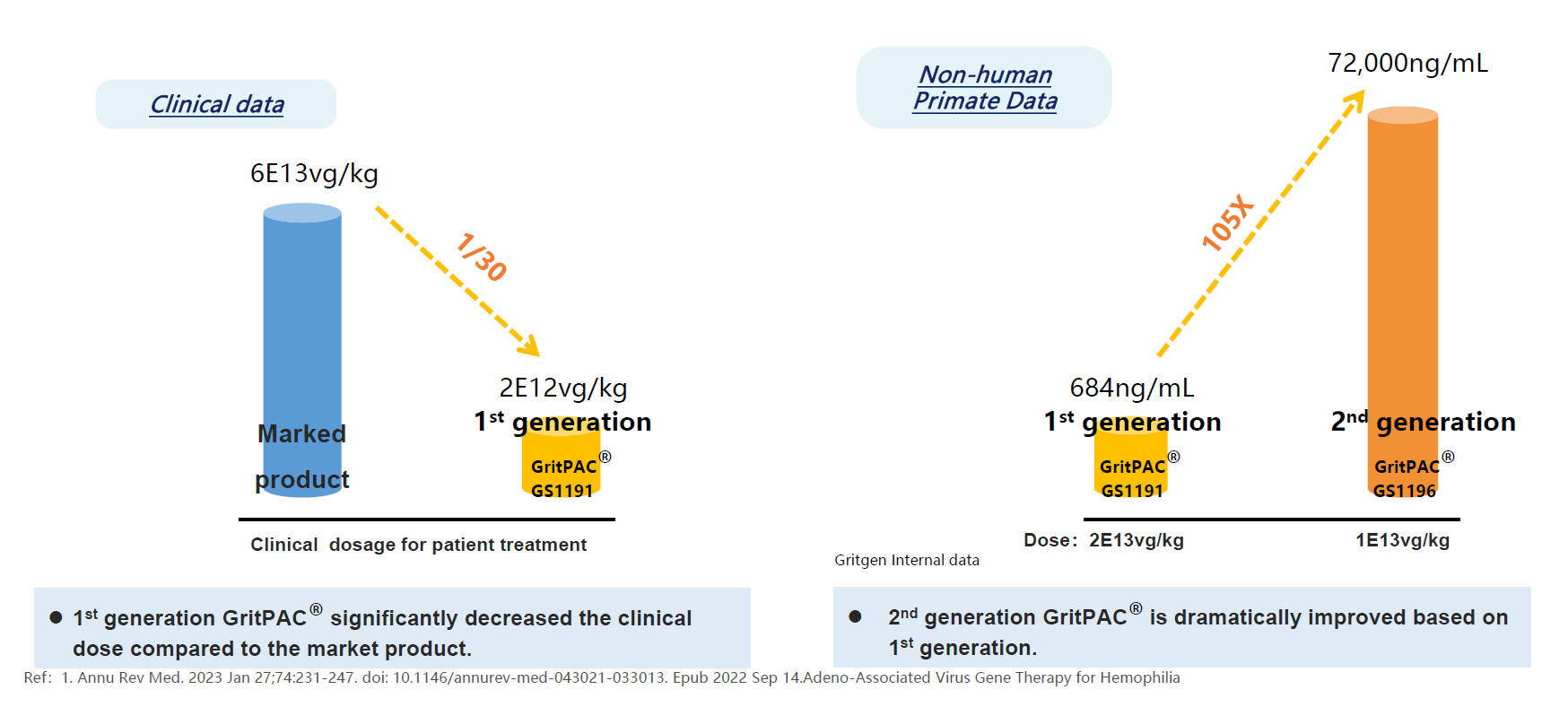
High Efficient Promotor Platform GritPAC®
High efficient promotor and enhancer system is able to increase the protein expression largely. Gritgen's GritPAC® platform has been demonstrated in non-human primate animal studies and clinical trials. GritPAC® makes the microgram per liter of protein expression in blood into reality, that broadens our pipeline significantly.
Next-generation AAV Capsid Platform GritOcul®
Gritgen has developed a next-generation, propriatary AAV capsid platform GritOcul®. Using the innovative capsid, gritgen is developing a long-lasting treatment for ophthamological disease.
-
01

HEK293 suspension
-
02

Three plasmids transfection
-
03

Lysis and harvest
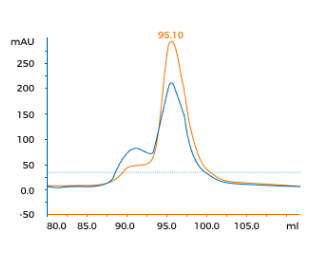
Upstream yield
-
01

Clarification
-
02

Column chromatography(affinity and anion exchange chromatography)
-
03

Ultrafiltration
-
04

Vertical filtration
-
05

Formulation
-
01

Cell thawing
-
02

Cell expansion in shake flask
-
03

Scale-up cultivation in fermenter
-
04

Cell harvest
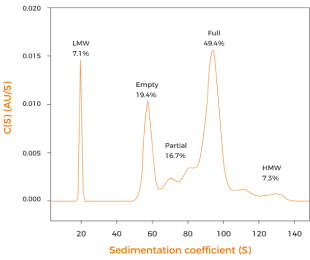
supercoiled ratio after fermentation
-
01

Continuous lysis
-
02

Column chromatography
-
03

Ultrafiltration
Integrated quality control system, with full compliance on regulatory requirements and clarified release specifications, ensure the manufacturing capability to meet clinical and commercial demands.


-
Aseptic filling of drug product in isolator



-
Cryopreservation



-
Large scale
-
High capacity
-
Compliance
-
8,600sq.m.
GMP facility
-
100L
Plasmid production line
-
2X500L
AAV production lines
















![comimg02638[1].png](/Public/Uploads/uploadfile/images/20230420/comimg02638[1].png)